
- Home
- Neurosurgery
- Durasis™ Dural Graft
Durasis™ Dural Graft
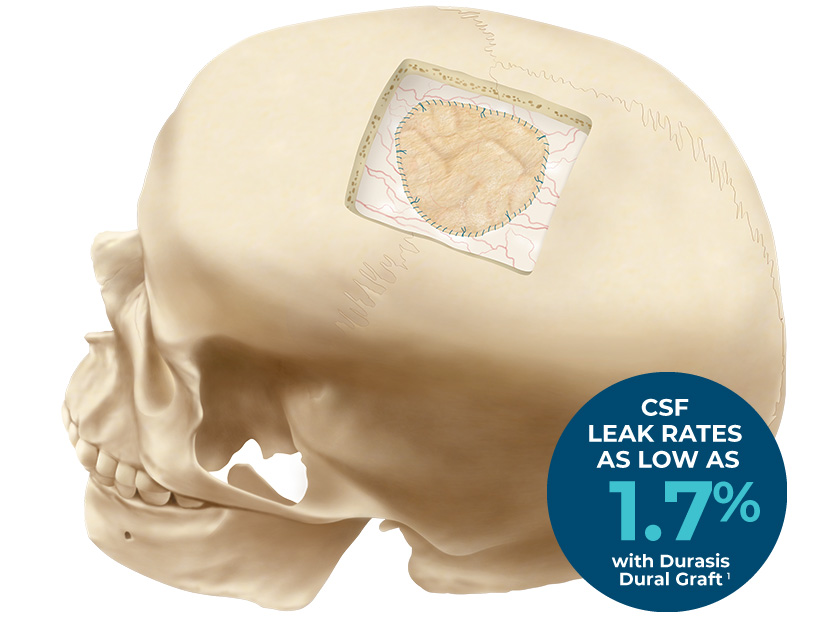
Seal the Deal with an Intact Biological Graft.
DURASIS™ DURAL GRAFT
The Durasis Dural Graft is intended for use as a dura substitute for repairing dura mater. 
Nexsis Biosciences is the exclusive U.S. and Canadian supplier of the Durasis Dural Graft (formerly Cook Biotech’s Biodesign® Dural Graft).
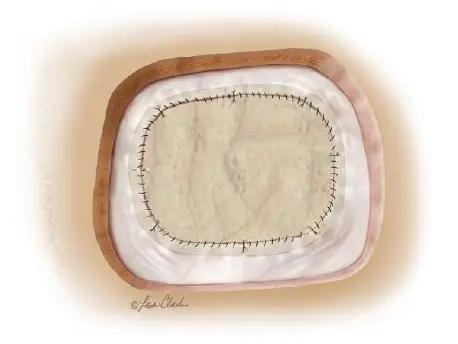
Durasis™ Dural Graft
STUDY #1
Durasis Dural Graft Yields High Success Rate as Dural Substitute
| Surgical Site | Number of Patients |
|---|---|
| Posterior fossa | 40 |
| Frontal | 6 |
| Spinal: cervical | 4 |
| Spinal: thoracic & lumbar | 4 |
| Frontal/temporal or temporal | 3 |
| Parietal/occipital or parietal | 2 |
| Neurological diagnosis | Number of Patients |
|---|---|
| Chiari malformation | 32 |
| Tumor/meningioma | 18 |
| Aneurysm | 3 |
| Spinal cord tethering | 3 |
| Other | 3 |
Results: Chiari malformation Type I decompression (32 patients) and tumor resection (18 patients) were the most common procedures performed, with 81% of SIS grafts implanted in the posterior fossa or spine. There was one case of a CSF leak (1.7%), two cases of wound infection (3.4%), and no cases of bacterial meningitis (0%) in the 58 patients available for follow up. In both cases of wound infection, the SIS graft acted as a barrier to infection and was not removed. Intraoperatively, a watertight seal was achieved in all 59 cases. On follow-up imaging available in 27 patients there was no evidence of any adverse reaction to the graft or of cerebral inflammation.Conclusions: This clinical evaluation of the SIS dural substitute demonstrated that rates of common complications associated with the use of dural substitutes compare favorably to experience reported in the literature with other dural substitute products. There was no evidence of scar tissue formation or encapsulation of the SIS dural substitute. The SIS dural substitute was judged to have excellent handling characteristics, and was associated with a very high rate of device and procedural success. Lack of adverse reactions to the graft, a favorable safety profile, and clinical efficacy all indicate the utility of this material as an alternative for the repair of dural defects.
STUDY #2
Retrospective Study Confirms Performance of Biodesign Grafts for Transcranial Dura Mater Repair
Purpose: The purpose of this study was to assess the real-world performance of the Durasis graft as a dura substitute for repairing dura mater. This report specifically analyzes a subset of patients who underwent transcranial dura mater repairs with the Durasis graft.
Endpoints: The primary endpoint was the postoperative integrity of the graft 1 month after surgery as defined by the lack of need to return to the operating room to repair a cerebrospinal fluid (CSF) leak. Secondary endpoints included postoperative graft integrity at the last available patient follow-up visit (with a minimum of 6 months follow-up) and the assessment of adverse events for normal, widespread use of the graft in the short-term postoperative period. Data were collected from 81 patients at Toronto Western Hospital, Toronto, Ontario, Canada.
| Primary Endpoints | n/N (percent) |
|---|---|
| CSF leak that required a return to the operating room (short-term follow-up, median 46 days) | 0/81 (0%) |
| Postoperative CNS infection | 0/81 (0%) |
| Required lumbar drain placement | 0/81 (0%) |
| Secondary Endpoints | n/N (percent) |
|---|---|
| CSF leak that required a return to the operating room (mid-term follow-up, median 175 days) | 0/72 (0%) |
Study Results: No unexpected or serious adverse events were reported among the 81 patients in the study, and no patients examined were returned to the operating room for a postoperative CSF leak. Of the 10 patients reporting complications, CSF leak (not requiring reoperation) (n=2, 2.5%), and wound infection(n=2, 2.5%) were the most frequently reported complications. None of these complications were considered unexpected or serious. They were also not reported to be device related.
Summary
Durasis grafts support the repair of dura mater by providing a conducive environment in which the body’s cells can attach, proliferate, and differentiate to restore organized, remodeled tissue. At the same time, the graft is slowly replaced during the normal process of collagen turnover such that no graft material remains after this process is complete.
Compared to other surgical treatments of dura mater repair, there are several benefits associated with the use of Durasis SIS.
Identified benefits of Durasis SIS grafts in dura mater repair
- No donor site required and no donor site morbidity
- Easy to handle and suture, allowing for a watertight seal
- Provides the strength required for a durable repair
- Functions as a scaffold for the ingrowth of host cells for a natural repair
- Produces little to no scar formation
- Does not swell upon hydration
- Provides a leak-free repair
Logo

